SWITCH: Results published
17.06.2024 – Results of a global trial on decompressive craniectomy in people with severe intracranial haemorrhages were published in The Lancet.
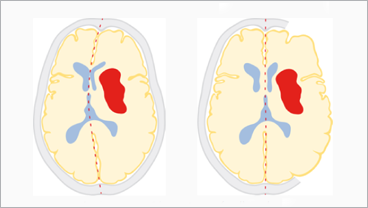
Treatment of people with severe intracranial haemorrhage is a major unresolved issue in stroke management, in particular with a bleeding into the deep structures of the brain. The randomized controlled trial SWITCH was designed to assess whether a surgical intervention – the decompressive craniectomy – would reduce severe handicap and mortality in these people.
The SWITCH trial enrolled 201 adults (18-75 years) with severe deep intracerebral haemorrhage who were randomly assigned to decompressive craniectomy plus best medical treatment or best medical treatment alone. 42 (44%) of participants receiving the decompressive craniectomy were severely disabled or deceased after 6 months, compared to 55 (58%) in the group receiving only medical treatment, resulting in an absolute risk reduction of 13% in the surgery group (95% confidence interval 0 to 26%, p-value 0.057). No evidence for a difference in adverse events was found.
The authors concluded that decompressive surgery plus best medical treatment might be superior to best medical treatment alone and that decompressive craniectomy is a potential treatment option for people with severe deep intracerebral haemorrhage. However, the evidence was weak and the findings may not universally apply to all types of intracerebral haemorrhage. Both treatment groups still exhibited high rates of severe disability and death.
The trial was led by the Department of Neurology of the University Hospital Bern. The DCR was involved in the planning and design of the study, provided monitoring and central data monitoring, and performed the statistical analysis.
Link to the publication:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)00702-5/fulltext